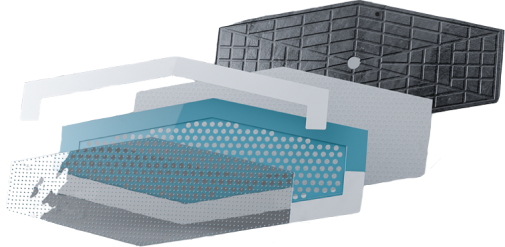
We are the full-cycle technology company that develops and manufactures soluble microneedle based medical devices and cosmetics for skin rejuvenation, wound healing, and intra-wound drug delivery


Soluble micronedles
Our microneedles penetrate only the outer layers of skin and application can be done at home without any medical training.
Easy to Use, Comfortable and Pain-Free

Microneedles can deliver any water-soluble substances including peptides, monoclonal antibodies, and DNA/RNA fragments.
Wide Choice of Active Ingredients

No requirement for refrigeration. No added preservative or stabilizers
No extra Preservatives or Stabilizers

Microneedles are intended for single use only, with no risk of contamination from shared use
Totally Safe

Problems
Almost everyone has experienced different degrees of skin damage, ranging from cosmetic imperfections and scarring caused by surgery and trauma, to chronic wounds that are difficult to heal. Furthermore, there is a large number of people who suffer from certain "lifestyle" diseases, for whom the condition of their skin is a determinant of the quality of life and its duration.
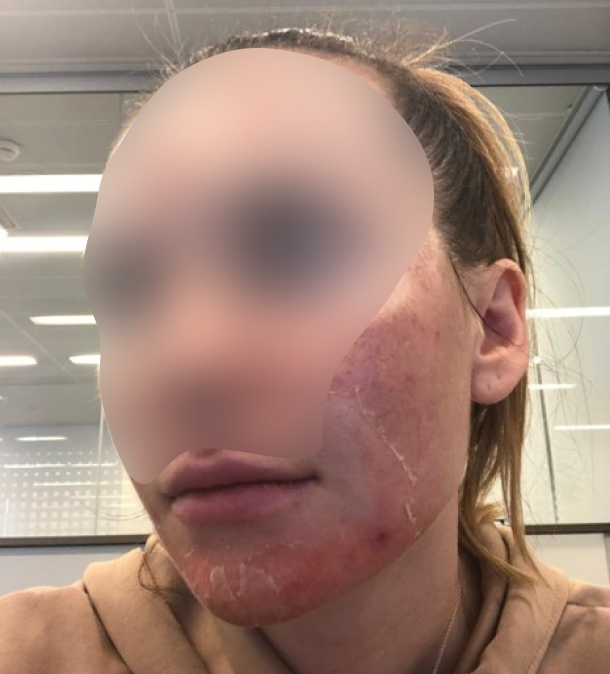
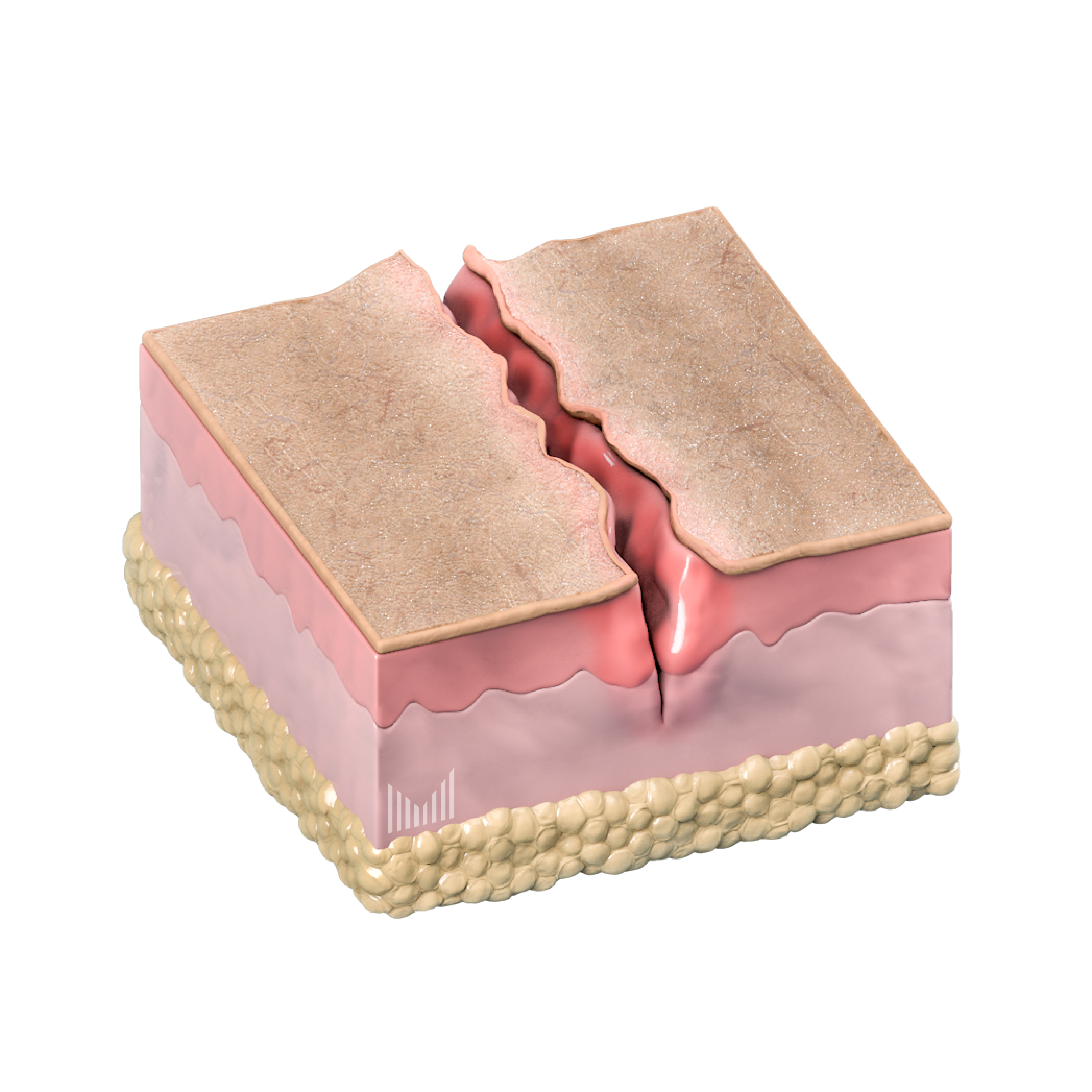
Our solution: epidermal dissolving microneedles
Stratum Corneum
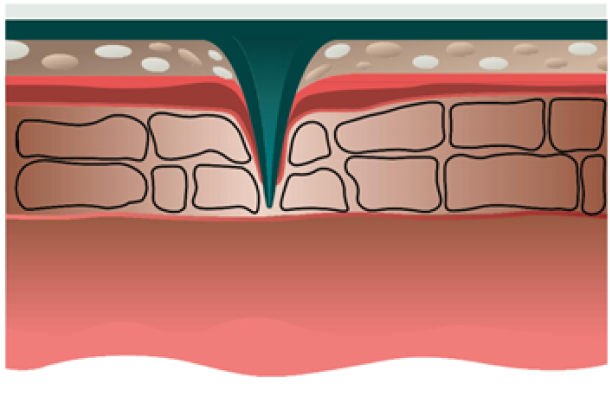
Stratum Lucidum/Stratum Granulosum
Stratum Basale
Dermis
1.
2.
3.
4.
1.
2.
3.
4.
recommended procedure time
25 min
>50%
of microneedle array are dissolved by skin enzymes
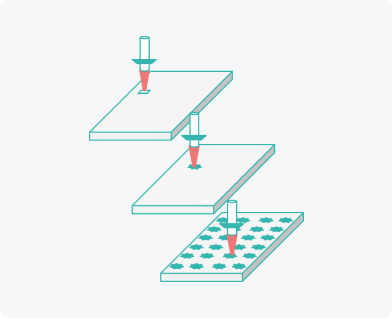
Production technology
We developed and patented our own equipment and production process of soluble microneedle arrays
Creating individual needle moulds using laser beam
1
2
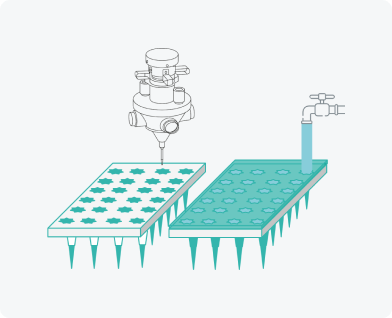
Filling moulds
Rapid implementation from R&D to the final product. Minimal limitation on size and shape.
3
Aerosol spraying of modifying additives
Rapid scaling of production. Wide variety of microneedle arrays
4
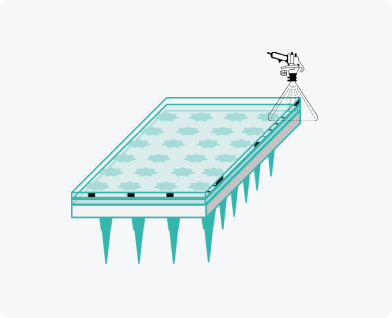
Adhesive film application
Compact and eco-friendly production

We currently produce up to 240 thousand items per month. We can scale up to 1 million items in 8 months


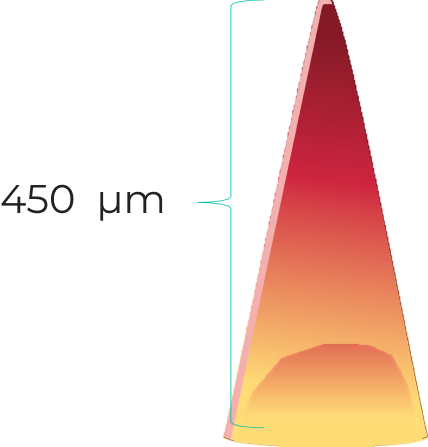
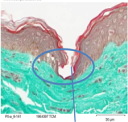
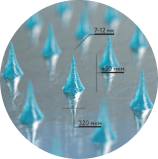
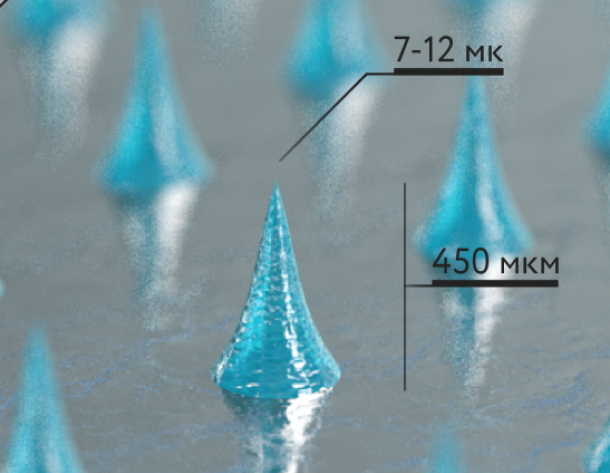
Our microneedles penetrate the skin to the depth of 30-50 μm and deliver active ingredients precisely into epidermis, ensuring their fast dissolution within 10 - 30 minutes.
Proven Efficacy
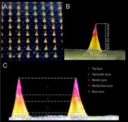
FDA submission Q202044 Class II (2) / De Novo and 510(k) Devices
Microneedles penetration depth is limited by the basal membrance
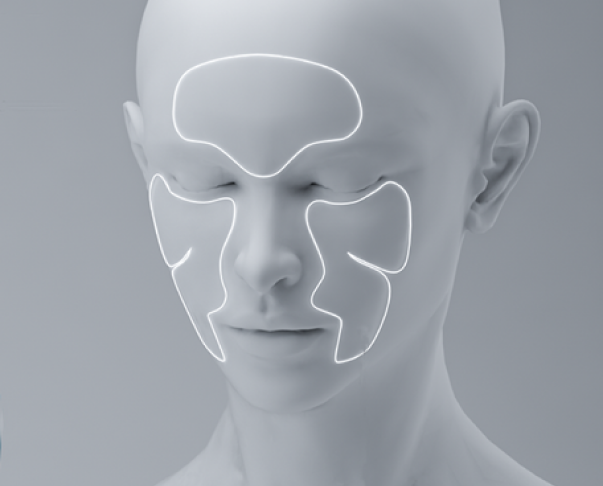
Key issues and platforms
Skin aging, hypersensitivity, puffiness
Cosmetic defects

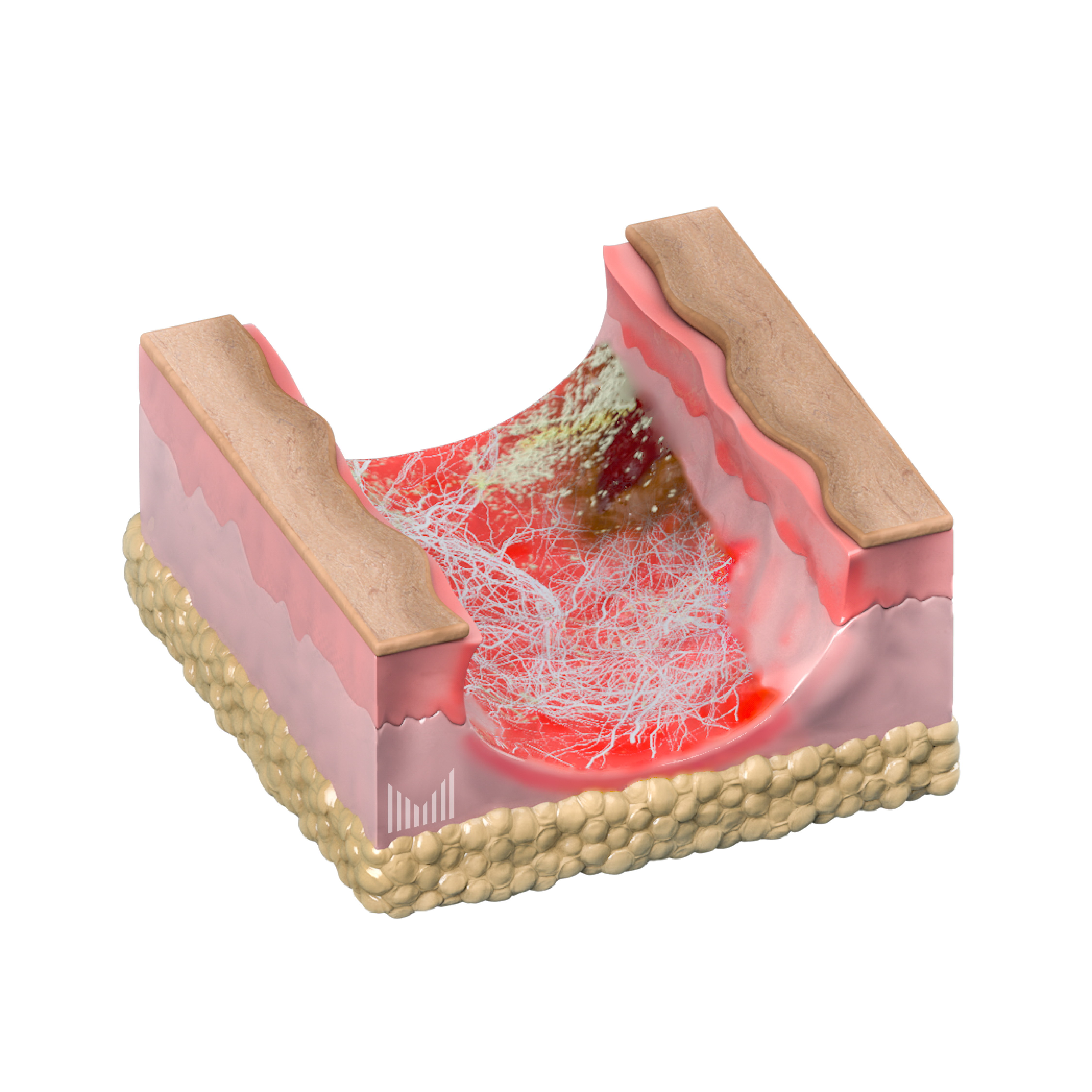
Injection of large-molecular pharmacutical compounds directly under wound surfaces with a wide area of active interaction
Intra-wound delivery
Cosmetic

Combined Medical device with pharmaceutical substance

Medical device (EU class IIa -III)
Skin rejuvenation in dermatology
Skin restoration after invasive cosmetic procedures, skin maceration, microtraumas, chapped skin, scarring prevention

Skin restoration in surgery
Skin restoration after surgical procedures, wound contamination prevention after open-chest surgery
Treatment of chronic wounds
Treating burns, chronic wounds therapy (eczema, diabetic foot, ulcerative lesion)
Microneedle applicator for skin rehabilitation after dermatological procedures (laser ablation, chemical peeling) EDMN-D
The protective film protects the adhesive layer around the edges of the applicator. The film is peeled off before applying the applicator to the skin
Protective Film

Made of soft material with polyacrylate adhesive layer
Back
Hyaluronic acid base converging into microneedles
Base
Microneedles made of polymerized hyaluronic acid with peptides/ They penetrate skin to the depth of the epidermis
Microneedles
1.
2.
3.
1.
2.
3.
Active Ingredients
The applicator design allows it to be applied comfortably all over the face. Providing a rejuvenating effect on all areas affected by invasive procedures.
Dipeptide Diaminibutyroyl Benzylamide Diacetate
Hyaluronic Acid (400 kDa)
Panthenol
Microneedle applicator for skin rehabilitation
after surgical procedures EDMN-S TM
after surgical procedures EDMN-S TM
The protective film protects the adhesive layer around the edges of the applicator. The film is peeled off before applying the applicator to the skin
Protective Film
The base of hyaluronic acid, on which the needles are fixed
Base
The dissolution modifer has a hole for the injection of solution. Channel systems lead to holes in the back
Dissolution Modifer
The Antiseptic modifier is made of hydroxy-methyl-cellulose. It contains colloida paprticles of nanosilver. It comes into contact with the scar after dissolving the microneedle array
Antiseptic Modifier
1.
2.
3.
4.
1.
2.
3.
4.
Microneedles
Microneedles made of polymerized hyaluronic acid containing peptides. They penetrate the skin to the depth of the epidermis.
Peptide anti-inflammatory complex
Hyaluronic Acid (400 kDa)
Panthenol
The shape enables a combination of applicators for applying to complex scars.
Active Ingredients
320 мкм
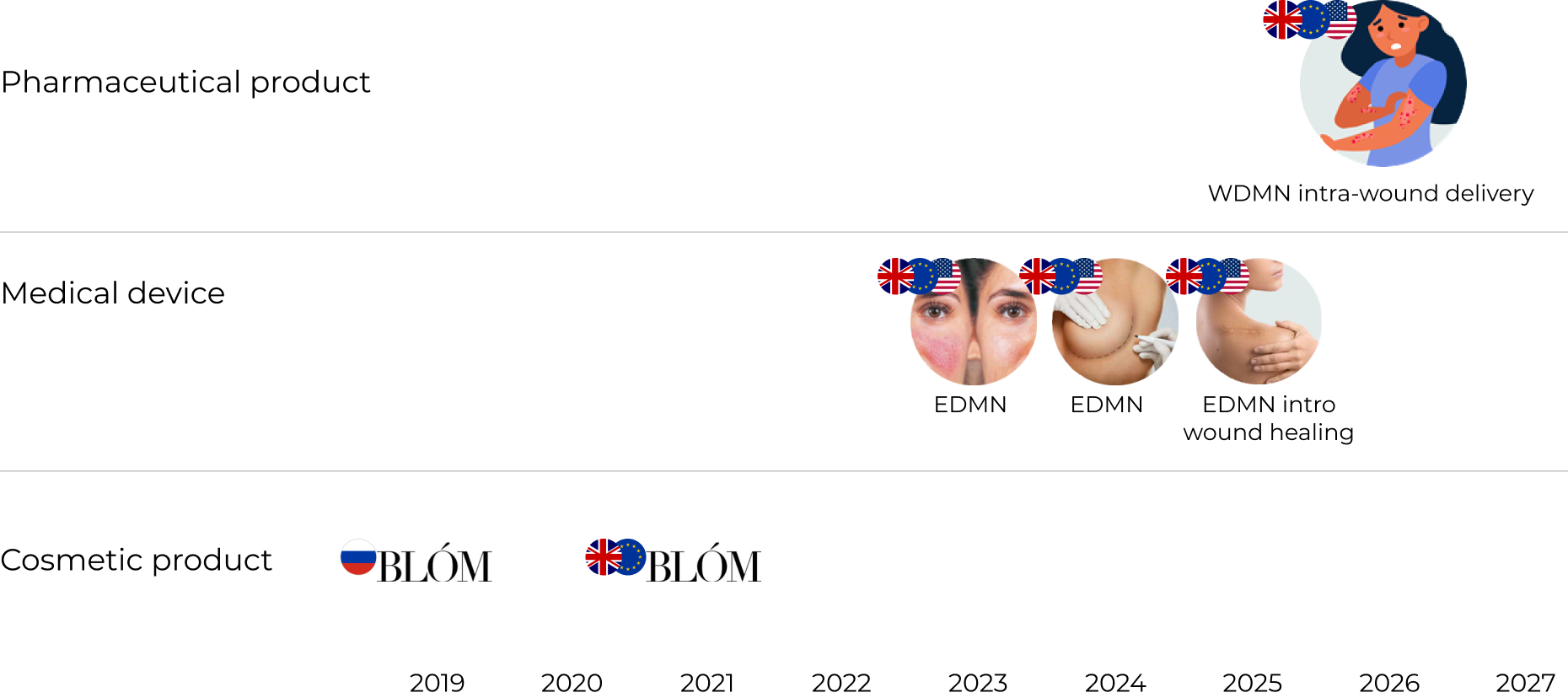
Pipeline

Market access
Team
Georgia Institute of Technology, Laboratory for Drug Delivery, internship with Mark Prausnitz
Dr. Vasilii Zvezdin
CO-FOUNDER, CSO
PHD in Medical Science, 68 scientific publications (Hirsch index 7), 13 patents
Head of the Biochemical and Nanosensory Diagnostics Laboratory of the Russian Federal Center for Public Health Risk Management
BSC, PHD, MBA
Mark Wickham

BUSINESS DEVELOPMENT
Over 30 years in the medical device industry in Europe and the USA
He has held senior management roles in large medical device companies Smith & Nephew plc, London International Group plc and Bespak plc, as well as having experience of founding companies, fund raising, and leading start-ups
BSc, PhD Biochemistry
Dr. Theresa Callaghan

SCIENTIFIC ADVISOR
Over 30 years in the international skincare and cosmetics industry gaining experience in leading roles - with LVMH, Unilever, Marks & Spencer, J&J, Evonik and proDERM
Over 120 publications and sits on the scientific editorial board of the International Journal of Cosmetic Science
PhD Plant Physiology
Nikita RADIONOV

STUDIES COORDINATOR
Skin scientist and cosmetic claims development expert
Guest Lecture at the University of Sunderland school of Pharmacy and Cosmetic Science (UK)
Our clinical experts
An international company that brings together professionals from all over the world to create solutions for the protection and regeneration of the skin.

Interested in a co-operation?
Please contact us here if you want to know more about our product
Contacts
Microneedles, Inc.
919 North Market Street, Suite 950 Wilmington, DE 19801, USA
919 North Market Street, Suite 950 Wilmington, DE 19801, USA

Head company
Francis Crick Ave, Trumpington, Cambridge CB2 0SL, UK
*Provisional address. It will be updated in November 2021
*Provisional address. It will be updated in November 2021

Center for Scientific Research of Wound Healing Processes
LLC "MicronidlIndustrial" 42 Bolshoy Boulevard, bldg. 1, Skolkovo Innovation Center, Moscow, 143026

Russian cosmetic division
LLC "MicronidlIndustrial" 14 Chelyuskintsev, Perm

Manufacture of cosmetic products
If you have any questions or would like further information please email us at: v.zvezdin@microneedle.tech
Leave your contacts and we will call you
It's totally free